- 작성일
- 2022.05.30
- 수정일
- 2022.05.30
- 작성자
- PEDAL
- 조회수
- 164
History of gases and plasmas – timeline
History of gases and plasmas – timeline
Use this timeline for a look at some of the historical aspects in the development of our understanding of gases and plasmas.
1644 – Invention of the barometer
Evangelista Torricelli (1608–1647), a student of Galileo, invents the barometer to prove that the Earth’s atmosphere has weight. His barometer is made by filling a glass tube, sealed at one end, with mercury and inverting into a bowl containing additional mercury.
1648 – Air pressure and altitude
Blaise Pascal (1623–1662) coins the term ‘pressure’ to indicate the weight of the air in the Earth’s atmosphere. Using a Torricelli barometer, he compares pressure at the base of a mountain with the top. He discovers air pressure decreases with height above base level.
1662 – Boyle’s Law
Robert Boyle (1627–1691) investigates the relationship between the volume of a fixed mass of gas, at constant temperature, and the pressure acting on it. He discovers that pressure is inversely proportional to volume. The Boyle’s Law formula is written as P1V1=P2V2.
1702 – Amontons’s Law
Guillaume Amontons (1663–1705) investigates the relationship between the pressure of a gas and its temperature. He discovers that, over the range from freezing to boiling water, the pressure increases by about one-third. Amontons’s Law can be written as P1/T1=P2/T2.
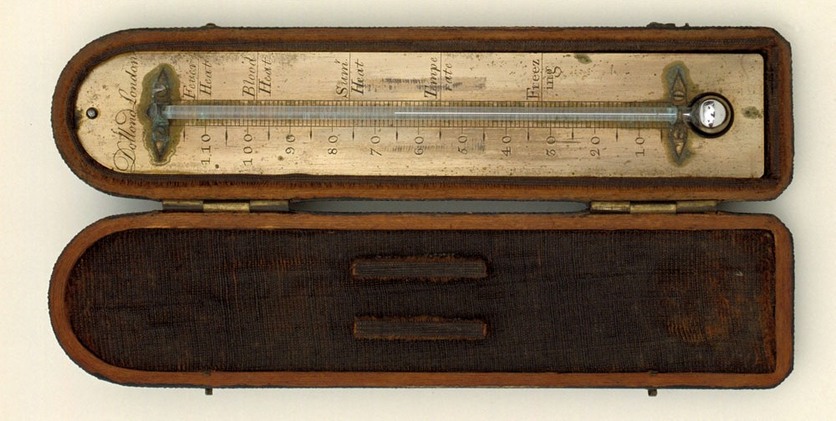
1714 – First mercury-in-glass thermometer
Daniel Gabriel Fahrenheit (1686–1736) designs a mercury-in-glass thermometer and develops a temperature scale now named after him. The three calibration points used are a freezing mixture of ice/salt (0°F), a water/ice mixture (32°F) and human body temperature (96°F).
1742 – Celsius temperature scale
Anders Celsius (1701–1744) develops a new temperature scale based on the freezing and boiling points of water. Celsius assigns a value of 100° for the freezing point and 0° for the boiling point. A year after his death, the scale is reversed to make it more practical.
1780 – James Six’s thermometer
James Six (1731–1793) invents a thermometer that allows maximum and minimum temperatures to be measured over a given time period. It proves to be most useful to meteorologists, and the basic design still remains in use today.
1787 – Charles’s Law
With the recent development of accurate thermometers, Jacques Charles (1746–1823) is able to investigate the relationship between the volume of a gas and its temperature. The modern interpretation of his findings states that V1/T1=V2/T2.
1801 – Dalton’s Law
John Dalton’s (1776–1844) interest in the weather motivates him to study gases. He is the first to observe that the pressure of a mixture of gases is the sum of the pressures of the various gases in the mixture. Dalton’s Law takes the form PT=P1+P2+P3+...
1803 – Henry’s Law
William Henry (1774–1836) discovers that the solubility of a gas in a liquid is directly proportional to the gas pressure. Henry’s Law takes the form Sg=kPg where Sg is the solubility of the gas and Pg is the pressure of the gas.
1808 – Gay-Lussac’s Law
Joseph Louis Gay-Lussac (1778–1850) formulates two gas laws. One, based on Amontons’s Law, relates the pressure of a gas and its temperature. The other, combining volumes law, relates to gaseous reactions and the volume ratios of reactant and product gases.
1811 – Avogadro’s Law
Amedeo Avogadro (1776–1856) extends the work of Gay-Lussac by proposing that gases consist of very small particles called molecules. His law states that equal volumes of gases under the same conditions of temperature and pressure contain equal numbers of molecules.
1834 – Ideal gas equation
Émile Clapeyron (1799–1864) is able to combine the laws of Boyle, Charles and Avogadro into a form known as the ideal gas equation. The modern form of this law states that PV=nRT, where n is the number of moles of gas and R the universal gas constant.
1846 – Graham’s Law
Thomas Graham (1805–1869) studies the way gases mix by diffusion as well as how they effuse through a semi-permeable barrier. He is able to show that the rate of effusion is inversely proportional to the square root of the molecular mass of the gas.
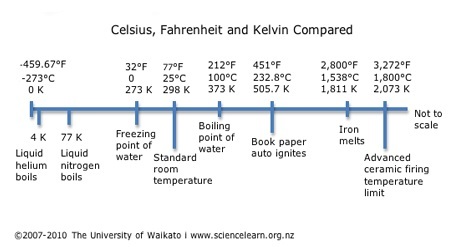
1848 – Kelvin temperature scale
Lord Kelvin (William Thomson) (1824–1907) recognises the need for a temperature scale whose zero point is equivalent to “infinite cold” or absolute zero. He calculates that absolute zero is equivalent to -273°C. The Kelvin scale is used in modern day science.
1857 – Kinetic-molecular theory
Rudolf Clausius (1822–1888) develops the kinetic theory of gases into a more sophisticated and systematic form. He introduces the concept of mean free path and links temperature to gas particle speed. Physical properties of gases can now be explained in particle terms.
1873 – Equation of state
Johannes van der Waals (1837–1923) realises gas laws and kinetic-molecular theory are based on an idealised gas. He develops an equation based on real gas behaviour that takes account of attractive forces between gas particles and the volume occupied by each particle.
1879 – The fourth state of matter
Sir William Crookes uses an assembly (today known as a Crookes tube) to form ionised air by passing an electrical discharge through gas at low pressure in a sealed tube. He labels it “radiant matter” because of its luminous quality.
1898 –- Kinetic theory analogy
William Ramsay (1852–1916), best known for his discovery of the noble gases, writes a discussion paper on the kinetic theory of gases and uses the analogy of football players being likened to ‘human molecules’ moving and colliding as the game plays out.
1924 – Existence of the ionosphere
Edward Appleton finally establishes the presence of an electrified conductive layer in the Earth’s upper atmosphere. This layer of ionised gas is given the name ‘ionosphere’ by Robert Watson-Watt in 1926.
1928 – Langmuir coins the term ‘plasma’
Irvine Langmuir gives the name ‘plasma’ to this fourth state of matter, as the way electrified gas carries high speed electrons and ions reminds him of the way blood plasma carries red and white blood cells. It replaces Crookes’s term ‘radiant matter’.
- 첨부파일
- 첨부파일이(가) 없습니다.