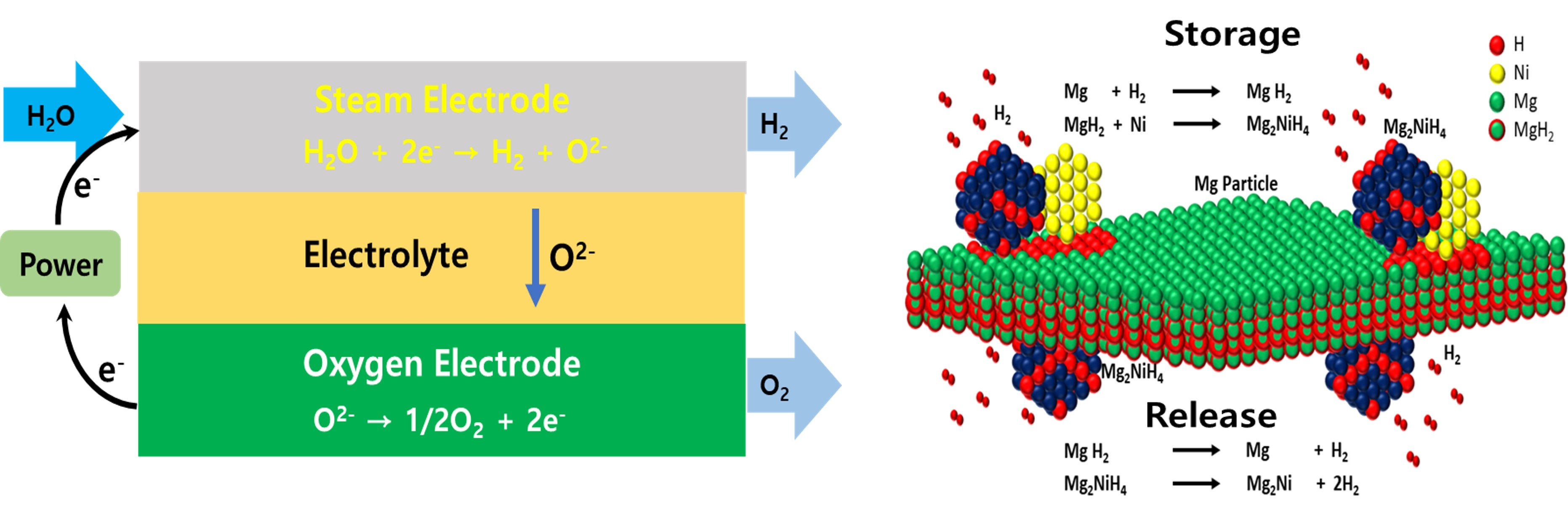
Solid oxide electrolyzer cell (SOEC) is a solid oxidation electrolysis device used for water electrolysis, a hydrogen production process, where water electrolysis refers to a technology that produces high-purity hydrogen from water through electrolysis. SOEC produces hydrogen by electrolyzing water vapor by inverting the process in which SOFC consumes hydrogen and oxygen to produce water and electricity. SOEC utilizes steam at a high temperature of up to 850 oC, which has the advantage of being able to produce hydrogen in a more efficient way because relatively less electricity is required at high temperatures than at low temperatures.
A metal hydride is a chemical storage system in which hydrogen is chemically stored on a metal in a solid state by an absorption process. Intermetallic compounds or alloys have shown the ability to absorb hydrogen under moderate pressure at low temperatures to form reversible solid metallic hydrogen compounds. Hydrogen absorbed by a chemical reaction in a solid medium can be stored at high density in the form of metal hydrides at room temperature and low pressure, which is why it is recognized as safer than other storage methods.
Our group is engaged in developing novel electrode materials for SOEC as well as metal alloys for hydrogen storage. Our group is also involved in acquiring a fundamental understanding of degradation mechanism.